Owing to its promising healing and restorative properties, Ginseng is among the most commonly used herbal medicines. It is derived from a plant, Panax ginseng, which has been used for centuries in Asia for therapeutic purposes. The plant can be classified into three depending on how long they are grown. They can either be red, white or fresh. Fresh ginseng is one that is harvested 4 years, while white ginseng is harvested between 4–6 years and red ginseng is harvested after 6 or more years (1). Ginseng contains active compounds known as ginsenosides through which they exhibit their therapeutic properties. Ginsenosides are categorized into two main classes based on the structure, e.g., the protopanaxadiol- and protopanaxatriol-families. As a major metabolite of protopanaxadiol type ginsenosides, compound K (CK). The pharmacological benefits of Ginseng are quite diverse. The herb has been demonstrated to be effective in the management of cancer, diabetes, cardiovascular diseases and have been used for promoting immune function, central nervous system (CNS) function, relieving stress, and for its antioxidant activities. This article explores the various benefits of Ginseng especially its metabolite compound K.

Potent antioxidant that reduces inflammation
Inflammation is the body’s natural response to noxious stimuli and disease. It is often accompanied by pain which can be devastating. Compound K has been shown to effectively reduce pain by enhancing antioxidant activity (2). In a study conducted to investigate the effects of having 18 young males take Korean red ginseng extract for seven days, it was demonstrated that taking athletes take 2 grams of Korean red ginseng extract three times per day resulted in the reduction of certain inflammatory markers (3). Diseases such as cancers often have their underlying basic pathology as inflammation. It is for this reason that several studies have been conducted to investigate the effects of compound K on cancer patients.

Improvement of brain functioning
In the recent past, several studies have been published with regards to the effects of ginseng and ginsenosides on central nervous system (CNS) disorders. While the majority believe CNS diseases to be confined to traditional target diseases such as stress and ischemia, neurological diseases expand into more recently acknowledged neurological and psychiatric disorders including Alzheimer’s disease (AD) and attention deficit hyperactivity disorder (ADHD). Some of these conditions such as Alzheimer’s disease are often accompanied by a reduction in brain functioning. Studies have shown that Ginseng and ginsenosides can help improve learning and memory (4). Ginsenosides affects neurotransmission of acetylcholine and gamma-aminobutyric acid (GABA) by a mechanism involving the regulation of the expression of synthetic enzymes, neurotransmitter release as well as the signaling pathways involved in the particular neurotransmitter systems (5). A study published in the sage journal showed that those study participants who took 200 mg of Panax ginseng had an improvement in mental performance (6).

Improvement of cardiovascular health
Among the major causes of morbidity and mortality in the United States is cardiovascular disease. Ginseng has been used widely by those at a risk of developing various cardiovascular diseases such as hypertension and hypercholesterolemia. Hypertension can lead to myocardial ischemia which often leads to the production of reactive oxygen species (7). Ginsenosides such as compound K have been shown to protect from myocardial reperfusion injury by increasing 6-keto-prostaglandin F1α production and decreasing lipid peroxidation (8). This helps alleviate the effects of the reactive oxygen species which often lead to endothelial dysfunction.

Skin protective effects of compound K
Among the most popular benefits of compound K is the role it plays in the management of skin disorders as well as its ability to act as an anti-aging agent. The aging of the skin is influenced by several genetic and environmental factors such as UV light and pollution which often leads to the hyperpigmentation of the skin, the loss of skin elasticity, dryness, and wrinkles. UV radiation has been shown to be the main cause of aging as it alters dermal connective tissues resulting in the degradation and reduction of collagen (9). It does this by activating particular matrix metalloproteinase (MMP) family members that mediate collagen degradation particularly in photoaged skin. It also leads to dryness which causes the barrier function of the skin to decline (10).
Studies have shown that compound K, an active metabolite of Ginseng, has anti-aging properties. These studies have shown the compound K activates skin metabolism resulting in increased blood flow and cell proliferation that leads to the regeneration of skin cells. One particular study established that dermal fibroblast express matrix metalloproteinase-1 (MMP-1) by exposure to both UVA and B. The study also showed that by administering compound K, collagen production increased while MMP-1 activity decreased (11).
In addition to its anti-aging properties, compound K has been shown to be beneficial in the management of several other dermatological conditions such as atopic eczema and dermatitis (12). These conditions are caused by a number of chemical agents such as proteases, cytokines, prostaglandins, histamine, neuropeptide substance P, and bile salts, which act as pruritogens. They often present with itchiness and pruritus. A study published in the journal of pharmacological sciences in 2005 evaluated the antipruritic effect of ginsenoside rb1 and compound k in scratching behavior mouse models (13). This study showed that compound K treatment reduced scratching behaviors and skin vascular permeability activated by compound 48/80, substance P, and histamine.

The anticancer effects of compound K
Cancer is the second leading cause of mortality and morbidity in the United States (14). There are several modalities of management which are mostly conventional involving procedures such as chemotherapy, radiation, and surgery. Whilst these methods have proven to be effective, studies have shown the need for some adjuvant alternative therapies owing to the complex characteristics of human cancers. One such carcinoma for which alternative therapies have been advocated for is skin cancer. Melanomas have been shown to frequently acquire chemoresistance and often require to be complemented with other forms of therapy. Several studies conducted to prove the effect of compound K in the management of skin cancers have shown that compound K plays a role in slowing down disease progression (16).
Tumor progression is regulated by the elevation of ornithine decarboxylase (ODC), free radicals, reactive oxygen species (ROS), COX-2, and NF-κB activity. A published in the journal Carcinogenesis showed that pretreatment with compound K inhibited the TPA induced activity of COX-2 and ODC by interfering with extracellular signal-regulated kinase (ERK) and nuclear factor-κB (NF-kB) pathway (17). Aside from skin cancers, compound K has been shown to be potent in the management of other cancers such as acute myeloid leukemia in children. A study published in the journal cancer cell international in 2013 showed that compound K induces apoptosis which results in the death of acute myeloid leukemia cancerous cells (18).

The antifatigue effects of Compound K
Fatigue is defined as a condition of extreme tiredness which often results from a broad range of physical and mental unfitness including inattention, distraction, and drowsiness. Its main etiological factor is the depletion of energy due to disorders of the internal environment such as reduced blood sugar levels. The main underlying pathophysiology of fatigue has been shown to be the excessive generation of reactive oxygen species in which these species cause lipid peroxidation of the mitochondrial membrane (19). Studies have implicated damaged mitochondria to the primary cause of depleted energy reserves. Therefore, interventions that curb oxidative stress often relieve fatigue effectively. Several studies have shown that the compounds of Ginseng such as compound K exhibit antioxidative properties thereby lowering oxidative stress and causing high energy production in cells and could help with pain relief. A randomized, double-blinded, placebo-controlled trial published in the journal Nutrients showed that Panax ginseng had anti-fatigue effects in patients with idiopathic chronic fatigue (20).
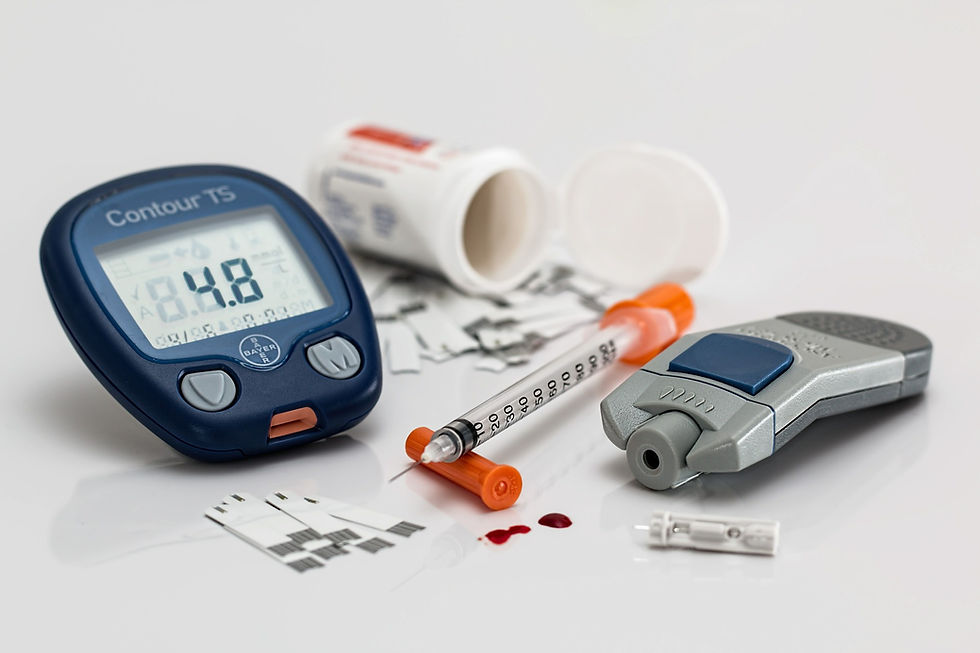
The glycemic control effects of compound K
Attaining glycemic control in diabetes mellitus has been shown to significantly improve outcomes in patients suffering from diabetes mellitus. Several studies conducted to investigate diabetes have shown that hyperglycemia is the principal cause of microvascular (retinopathy, neuropathy, nephropathy) and macrovascular (heart disease, stroke, amputations) complications suggesting effective blood glucose control as the key to preventing or reversing diabetic complications and improving quality of life in patients with diabetes. While conventional therapies have been effective in the management of hyperglycemia, studies have suggested the need for alternative therapies to complements these conventional treatments. American and Asian ginseng have been shown to improve pancreatic cell function, boost insulin production and enhance the uptake of blood sugar in tissues (21). A study conducted to investigate the glycemic effects of compound K on a group of 19 participants with diabetes mellitus showed that blood sugar control was achieved during the 12-weeks of study. The study also showed an 11% decrease in blood sugar levels, a 38% decrease in fasting insulin and a 33% increase in insulin sensitivity (22).

Hepatoprotective effects of compound K
Acute liver failure refers to the rare but often heterogeneous presentation of severe liver dysfunction that occurs in a patient with no preexisting liver damage. The condition has a high mortality and morbidity rate and is often a major cause of liver transplantation. Clinically, it is defined as the development of severe acute liver injury with encephalopathy and impaired synthetic function (INR of 1.5 or higher) in a patient without cirrhosis or preexisting liver disease and with an illness of fewer than 26 weeks duration (23). Among the major but rare causes of acute liver failure is acetaminophen toxicity which often occurs as a result of acetaminophen suicide or unintentional consumption of several tablets. To prevent this side effect of APAP, the protective effects of various agents against liver injury have been investigated. At present, the oxidative stress caused by N-acetyl-p-benzoquinone-imine (NAPQI),one of the paracetamol (APAP) metabolites, has been considered to be responsible for severe hepatotoxicity. While the mechanism of ginseng metabolites such as compound K remain unclear, several studies have shown that its role as an antioxidant can go a long way in reducing the hepatotoxic effects of acetaminophen (24).

Reproductive effects of compound K
Erectile dysfunction is a relatively common condition that affects approximately 30 to 50% of men aged 40 to 70 years (25). Majority of the cases have been attributed to smoking and obesity while approximately 20 % of the cases have been linked to psychological issues. Currently, erectile dysfunction is managed through oral medications, intra-penile therapies such as intra-urethral suppositories and intra-cavernous injections, and penile prosthesis injections (26). Increasingly, people suffering from this condition have been seeking alternative therapies. One particular herbal therapy that has been used by many is ginseng. Its efficacy in managing the condition has been attributed to the role it plays in reducing oxidative stress in blood vessels and tissues in the penis. This helps to restore normal function Studies conducted to investigate the role of the herb in the management of sexual dysfunction have demonstrated its effectiveness. A study published in the journal British journal of clinical pharmacology demonstrated that men treated with Korean red ginseng had a 60% improvement in ED symptoms, compared to 30% improvement produced by a medication used to treat ED (27).
SOURCES
Bao, L., Cai, X., Wang, J., Zhang, Y., Sun, B., & Li, Y. (2016). Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients, 8(12), 807. doi:10.3390/nu8120807
Chen, Y., Xu, Y., Zhu, Y., & Li, X. (2013). Anti-cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell International, 13(1), 24. doi:10.1186/1475-2867-13-24
Choi, J., Kim, T., Choi, T., & Lee, M. S. (2013). Ginseng for Health Care: A Systematic Review of Randomized Controlled Trials in Korean Literature. PLoS ONE, 8(4), e59978. doi:10.1371/journal.pone.0059978
Dong, K. K., Damaghi, N., Picart, S. D., Markova, N. G., Obayashi, K., Okano, Y., … Yarosh, D. B. (2008). UV-induced DNA damage initiates release of MMP-1 in human skin. Experimental Dermatology, 17(12), 1037-1044. doi:10.1111/j.1600-0625.2008.00747.x
Geng, J., Dong, J., Ni, H., Wu, T., Jiang, K., & Wang, G. (2009). Ginseng for cognition. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.cd007769
Igami, K., Shimojo, Y., Ito, H., Miyazaki, T., & Kashiwada, Y. (2014). Hepatoprotective effect of fermented ginseng and its major constituent compound K in a rat model of paracetamol (acetaminophen)-induced liver injury. Journal of Pharmacy and Pharmacology, 67(4), 565-572. doi:10.1111/jphp.12342
Jang, D., Lee, M. S., Shin, B., Lee, Y., & Ernst, E. (2008). Red ginseng for treating erectile dysfunction: a systematic review. British Journal of Clinical Pharmacology, 66(4), 444-450. doi:10.1111/j.1365-2125.2008.03236.x
Jin, X., Che, D., Zhang, Z., Yan, H., Jia, Z., & Jia, X. (2016). Ginseng consumption and risk of cancer: A meta-analysis. Journal of Ginseng Research, 40(3), 269-277.
doi:10.1016/j.jgr.2015.08.007
Jung, H. L., Kwak, H. E., Kim, S. S., Kim, Y. C., Lee, C. D., Byurn, H. K., & Kang, H. Y. (2011). Effects of Panax Ginseng Supplementation on Muscle Damage and Inflammation after Uphill Treadmill Running in Humans. The American Journal of Chinese Medicine, 39(03), 441-450. doi:10.1142/s0192415x11008944
Kim, E. H., & Kim, W. (2018). An Insight into Ginsenoside Metabolite Compound K as a Potential Tool for Skin Disorder. Evidence-Based Complementary and Alternative Medicine, 2018, 1-8. doi:10.1155/2018/8075870
Kim, E., Kim, D., Yoo, S., Hong, Y. H., Han, S. Y., Jeong, S., … Park, J. (2018). The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. Journal of Ginseng Research, 42(2), 218-224. doi:10.1016/j.jgr.2017.03.007
Kim, H. J., Kim, P., & Shin, C. Y. (2013). A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. Journal of Ginseng Research, 37(1), 8-29. doi:10.5142/jgr.2013.37.8
Kim, J. (2012). Cardiovascular Diseases and Panax ginseng: A Review on Molecular Mechanisms and Medical Applications. Journal of Ginseng Research, 36(1), 16-26. doi:10.5142/jgr.2012.36.1.16
Lee, J. (2004). Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol-type ginsenosides in mouse skin. Carcinogenesis, 26(2), 359-367. doi:10.1093/carcin/bgh313
Lee, C. H., & Kim, J. (2014). A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research, 38(3), 161-166. doi:10.1016/j.jgr.2014.03.001
Lee, D. C., & Lau, A. S. (2011). Effects of Panax ginseng on Tumor Necrosis Factor-α-Mediated Inflammation: A Mini-Review. Molecules, 16(4), 2802-2816. doi:10.3390/molecules16042802
Luo, J. Z., & Luo, L. (2009). Ginseng on Hyperglycemia: Effects and Mechanisms. Evidence-Based Complementary and Alternative Medicine, 6(4), 423-427. doi:10.1093/ecam/nem178
Mellinger, J. L., & Fontana, R. J. (2017). Management of Acute Liver Failure. Evidence-Based Critical Care, 551-560. doi:10.1007/978-3-319-43341-7_64
Park, B., Jung, H., Cho, Y., Lim, H., & Lim, C. (2012). Potentiation of antioxidative and anti-inflammatory properties of cultured wild ginseng root extract through probiotic fermentation. Journal of Pharmacy and Pharmacology, 65(3), 457-464. doi:10.1111/jphp.12004
Pyrsopoulos, N. T. (2018). Acute Liver Failure. Philadelphia: Elsevier.
Reay, J. L., Kennedy, D. O., & Scholey, A. B. (2005). Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. Journal of Psychopharmacology, 19(4), 357-365. doi:10.1177/0269881105053286
Vuksan, V., Sung, M., Sievenpiper, J. L., Stavro, P. M., Jenkins, A. L., Di Buono, M., … Naeem, A. (2008). Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutrition, Metabolism and Cardiovascular Diseases, 18(1), 46-56. doi:10.1016/j.numecd.2006.04.003
Wong, A. S., Che, C., & Leung, K. (2015). Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Natural Product Reports, 32(2), 256-272. doi:10.1039/c4np00080c
Yang, Y., Ren, C., Zhang, Y., & Wu, X. (2017). Ginseng: A Nonnegligible Natural Remedy for Healthy Aging. Aging and Disease, 8(6), 708. doi:10.14336/ad.2017.0707
Yao, H., Wan, J., Zeng, J., Huang, W., Sava‑Segal, C., Li, L., … Yuan, C. (2018). Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncology Letters. doi:10.3892/ol.2018.8414
Comments